The Electron-pair Geometry Around the Br Atom in Bro4- Is
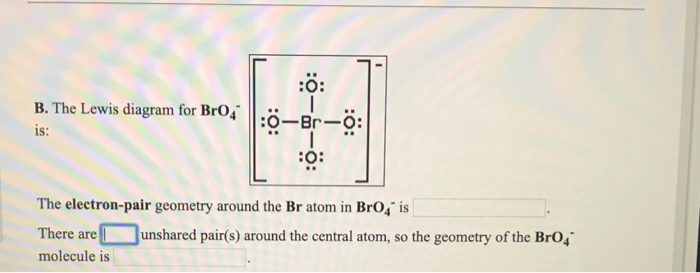
It is a conjugate base of a perbromic acid. The Lewis diagram for BrO is.
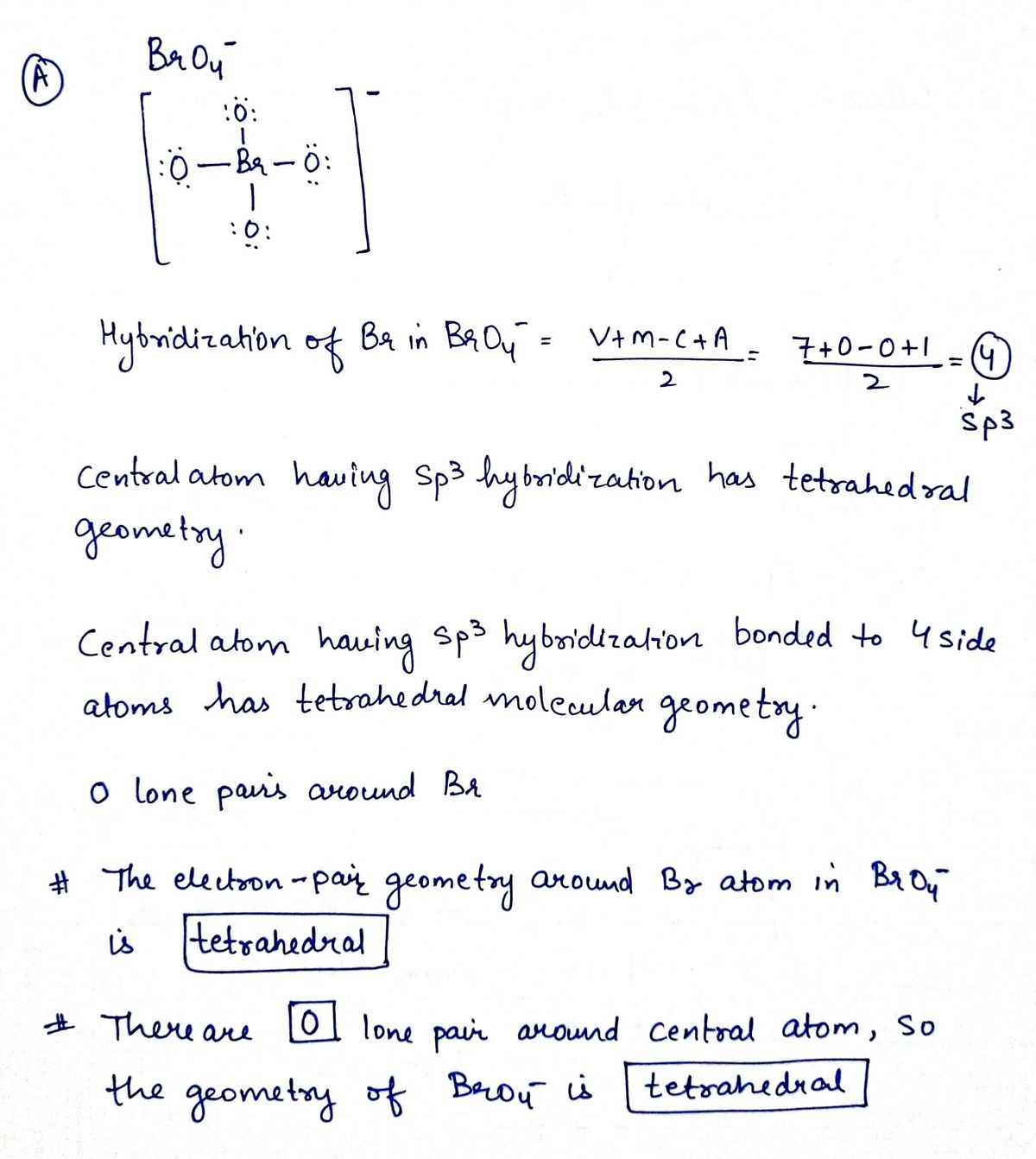
Answered O A The Lewis Diagram For Bro Is Bartleby
The molecular geometry on the other hand is Trigonal Pyramidal.

. There are four groups surrounding the bromine atom giving it a tetrahedral electron geometry. One single bonded O. The geometry of NOBr is bent and the molecule is polar.
To start Br atom has 7 valence electrons. There are no lone pairs around the central atom Br View the full answer. Terms in this set 43 POBr3 p IS THE.
4 of those electrons. He has taught science for over twenty years in the US. Br O3 -O- Charges and free electron pairs not drawn in the smiley above.
Electron pairs are defined as electrons in bonds lone pairs and occasionally a single unpaired electron. The electron-pair geometry for N in NOBr is trigonal planar. Find the electron configuration and ground state term symbol for a Co3.
The electron geometry Electronic Domain Geometry for PF3 is tetrahedral. Chem test 2 - molecular structure and electron-pair geometry. The Lewis structure of BrO 4 B r O 4 is given.
A Electron pair geometry of BrO4- is Tetrahedral. This molecule will be a transitory free radical with 1 unpaired electron. The remaining 4 valence electrons will be placed on bromine as lone pairs.
The electron-pair geometry around the Br atom in Brod is There are lone pairs around the central atom so the geometry of Bro is B. Molecular geometry is the name of the geometry used to describe the shape of a molecule. B is chemist and learning scientist who enjoys creating videos to help students with chemistry and other science topics.
What is the electron pair. Since BrO 4 has one bromine atom and four oxygen atoms so Valence electrons of one bromine atom 7 1 7 Valence electrons of four oxygen atoms 6 4 24. Up to 24 cash back What is the electron-pair geometry for br in brf4-1.
The hybridization of the Br atom is sp3. 4 Fs mean 4 Br-F bonds requiring 8 electrons. Is acetate trigonal planar.
Value 7 O ox. The various geometries are shown in the graphic on the upper left. The bromine atom is surrounded by 6 regions of electron density - four single bonds and 2 lone.
What is the electron-pair geometry for. The Lewis structure of acetic. The electron-pair geometry provides a guide to the bond angles of between a terminal-central.
What is the electron-pair geometry for P in PBr 4 _____ There are _____ lone pairs around the central atom so the geometry of PBr 4 is _____ B. Up to 24 cash back electron pair and molecular geometry is tetrahedral AX4. The electron-pair geometry around the Br atom in BrO4 is There are lone pairs around the.
The Lewis diagram for BrO4 15. Perbromate is a monovalent inorganic anion obtained by deprotonation of perbromic acidIt is a bromine oxoanion and a monovalent inorganic anion. A central atom with five pairs of bonding electron pairs is known as trigonal bipyramidal.
See full answer below. Perbromate BrO4- Br ox. It has the shape of three pairs in a plane at 120 angles the trigonal planar geometry and the.
This O has 3. Nonbonding domains lone pairs are symmetrically placed around central atom the molecule will be non polar Imagine a molecule MX2 in which both X atoms are bonded to M and M also has.
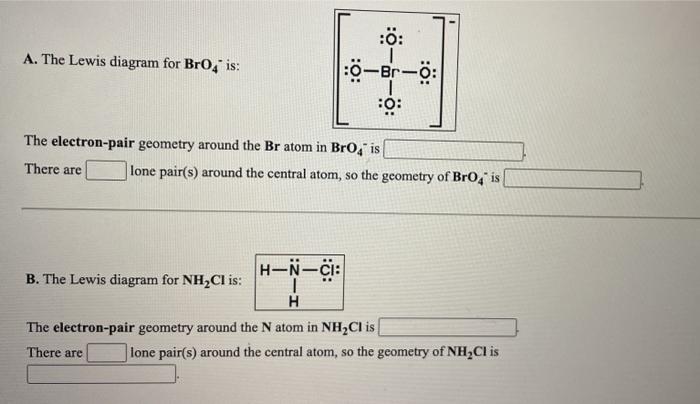
Solved A The Lewis Diagram For Bro4 Is 10 Br O The Chegg Com
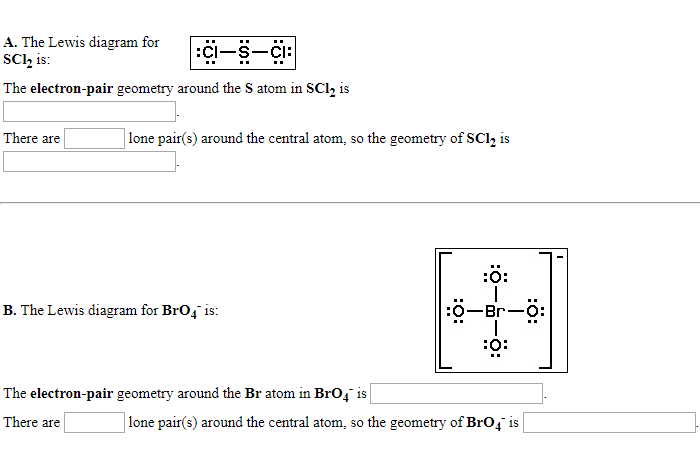
Solved A The Lewis Diagram For Sclh Is Ci S Ci The Chegg Com
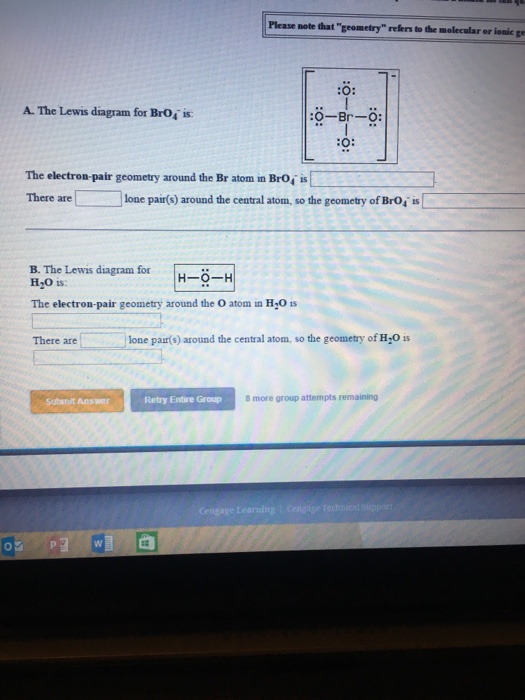
Solved The Lewis Diagram For Bro 4 Is The Electron Pair Chegg Com
No comments for "The Electron-pair Geometry Around the Br Atom in Bro4- Is"
Post a Comment